Einsteinium, 99Es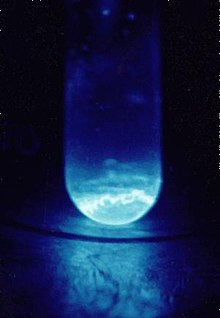 |
| Einsteinium |
|---|
| Pronunciation | (eyen-STY-nee-əm) |
|---|
| Appearance | siller-coloured |
|---|
| Mass number | [252] |
|---|
| Einsteinium in the periodic cairt |
|---|
|
|
| Atomic nummer (Z) | 99 |
|---|
| Group | group n/a |
|---|
| Period | period 7 |
|---|
| Block | f-block |
|---|
| Element category | Actinide |
|---|
| Electron confeeguration | [Rn] 5f11 7s2 |
|---|
| Electrons per shell | 2, 8, 18, 32, 29, 8, 2 |
|---|
| Pheesical properties |
|---|
| Phase at STP | solit |
|---|
| Meltin pynt | 1133 K (860 °C, 1580 °F) |
|---|
| Bylin pynt | 1269 K (996 °C, 1825 °F) (estimatit) |
|---|
| Density (near r.t.) | 8.84 g/cm3 |
|---|
| Atomic properties |
|---|
| Oxidation states | +2, +3, +4 |
|---|
| Electronegativity | Pauling scale: 1.3 |
|---|
| Ionisation energies | |
|---|
 Colour lines in a spectral rangeSpectral lines o einsteinium Colour lines in a spectral rangeSpectral lines o einsteinium |
| Ither properties |
|---|
| Naitural occurrence | synthetic |
|---|
| Creestal structur | face-centred cubic (fcc) |
|---|
| Magnetic orderin | paramagnetic |
|---|
| CAS Nummer | 7429-92-7 |
|---|
| History |
|---|
| Namin | after Albert Einstein |
|---|
| Diskivery | Lawrence Berkeley Naitional Laboratory (1952) |
|---|
| Main isotopes o einsteinium |
|---|
|
|
| | references |
| style="text-align:left"|
|
|
|
in
|
calc from C
|
diff
|
report
|
ref
|
| C
|
860
|
—
|
—
|
|
|
| K
|
1133
|
1130
|
3
|
delta
|
|
| F
|
1580
|
1580
|
0
|
|
|
| WD
|
String Module Error: Target string is empty ! 
|
|
|
|
| input
|
C: 860, K: 1133, F: 1580
|
| comment
|
|
| style="text-align:left"|
|
|
|
in
|
calc from C
|
diff
|
report
|
ref
|
| C
|
996
|
—
|
—
|
|
|
| K
|
1269
|
1269
|
0
|
|
|
| F
|
1825
|
1825
|
0
|
|
|
| WD
|
String Module Error: Target string is empty ! 
|
|
|
|
| input
|
C: 996, K: 1269, F: 1825
|
| comment
|
(estimatit)
|
References
Thir references will appear in the airticle, but this list appears anerly on this page.


