Actinium, 89Ac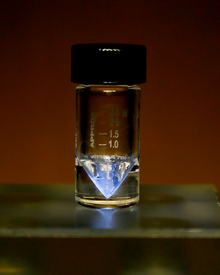 |
| Actinium |
|---|
| Pronunciation | (ak-TIN-nee-əm) |
|---|
| Appearance | sillery-white, glowin wi an eerie blue licht;[1] sometimes with a golden cast[2] |
|---|
| Mass number | [227] |
|---|
| Actinium in the periodic cairt |
|---|
|
|
| Atomic nummer (Z) | 89 |
|---|
| Group | group 3 |
|---|
| Period | period 7 |
|---|
| Block | f-block |
|---|
| Element category | Actinide |
|---|
| Electron confeeguration | [Rn] 6d1 7s2 |
|---|
| Electrons per shell | 2, 8, 18, 32, 18, 9, 2 |
|---|
| Pheesical properties |
|---|
| Phase at STP | solit |
|---|
| Meltin pynt | 1500 K (1227 °C, 2240 °F) (estimatit)[2] |
|---|
| Bylin pynt | 3500±300 K (3200±300 °C, 5800±500 °F) (extrapolatit)[2] |
|---|
| Density (near r.t.) | 10 g/cm3 |
|---|
| Heat o fusion | 14 kJ/mol |
|---|
| Heat o vapourisation | 400 kJ/mol |
|---|
| Molar heat capacity | 27.2 J/(mol·K) |
|---|
| Atomic properties |
|---|
| Oxidation states | +2, +3 strangly basic |
|---|
| Electronegativity | Pauling scale: 1.1 |
|---|
| Ionisation energies | - 1st: 499 kJ/mol
- 2nd: 1170 kJ/mol
-
|
|---|
| Covalent radius | 215 pm |
|---|
 Colour lines in a spectral rangeSpectral lines o actinium Colour lines in a spectral rangeSpectral lines o actinium |
| Ither properties |
|---|
| Creestal structur | face-centred cubic |
|---|
| Thermal conductivity | 12 W/(m·K) |
|---|
| CAS Nummer | 7440-34-8 |
|---|
| History |
|---|
| Diskivery an first isolation | Friedrich Oskar Giesel (1902) |
|---|
| Main isotopes o actinium |
|---|
|
|
| | references |
| style="text-align:left"|
|
|
|
in
|
calc from C
|
diff
|
report
|
ref
|
| C
|
1227
|
—
|
—
|
|
|
| K
|
1500
|
1500
|
0
|
|
|
| F
|
2240
|
2241
|
-1
|
delta
|
|
| WD
|
String Module Error: Target string is empty ! 
|
|
|
|
| input
|
C: 1227, K: 1500, F: 2240
|
| comment
|
(estimatit)[2]
|
| style="text-align:left"|
|
|
|
in
|
calc from C
|
diff
|
report
|
ref
|
| C
|
3200
|
—
|
—
|
|
|
| K
|
3500
|
3470
|
30
|
delta
|
|
| F
|
5800
|
5790
|
10
|
delta
|
|
| WD
|
String Module Error: Target string is empty ! 
|
|
|
|
| input
|
C: 3200±300, K: 3500±300, F: 5800±500
|
| comment
|
(extrapolatit)[2]
|
References
Thir references will appear in the airticle, but this list appears anerly on this page.
- ↑ Wall, Greg (8 September 2003). "C&EN: It's Elemental: The Periodic Table - Actinium". C&EN: It's Elemental: The Periodic Table. Chemical and Engineering News. Retrieved 2 Juin 2011.
- ↑ a b c

