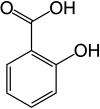
Salicylic acid
Salicylic acid (frae Laitin salix, willow tree, frae the bark o which the substance uised tae be obtained) is a monohydroxybenzoic acid, a teep o phenolic acid an a beta hydroxy acid. It haes the formula C7H6O3. This colourless crystalline organic acid is widely uised in organic synthesis an functions as a plant hormone. It is derived frae the metabolism o salicin. In addeetion tae bein an important active metabolite o aspirin (acetylsalicylic acid), which acts in pairt as a prodrug tae salicylic acid, it is probably best kent for its uise as a key ingredient in topical anti-acne products. The sauts an esters o salicylic acid are kent as salicylates.
| |||

| |||
| Names | |||
|---|---|---|---|
| IUPAC name
2-Hydroxybenzoic acid
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| EC Nummer | 200-712-3 | ||
| KEGG | |||
PubChem CID
|
|||
| RTECS nummer | VO0525000 | ||
| UNII | |||
| |||
| |||
| Properties | |||
| C7H6O3 | |||
| Molar mass | 138.12 g·mol−1 | ||
| Appearance | colourless tae white crystals | ||
| Odour | odorless | ||
| Density | 1.443 g/cm3 (20 °C)[1] | ||
| Meltin pynt | 158.6 °C (317.5 °F; 431.8 K) | ||
| Bylin pynt | 200 °C (392 °F; 473 K) decomposes[2] 211 °C (412 °F; 484 K) at 20 mmHg[1] | ||
| sublimes at 76 °C[3] | |||
| 1.24 g/L (0 °C) 2.48 g/L (25 °C) 4.14 g/L (40 °C) 17.41 g/L (75 °C)[2] 77.79 g/L (100 °C)[4] | |||
| Solubility | soluble in ether, CCl4, benzene, propanol, acetone, ethanol, ile o turpentine, toluene | ||
| Solubility in benzene | 0.46 g/100 g (11.7 °C) 0.775 g/100 g (25 °C) 0.991 g/100 g (30.5 °C) 2.38 g/100 g (49.4 °C) 4.4 g/100 g (64.2 °C)[2][4] | ||
| Solubility in chloroform | 2.22 g/100 mL (25 °C)[4] 2.31 g/100 mL (30.5 °C)[2] | ||
| Solubility in methanol | 40.67 g/100 g (−3 °C) 62.48 g/100 g (21 °C)[2] | ||
| Solubility in olive ile | 2.43 g/100 g (23 °C)[2] | ||
| Solubility in acetone | 39.6 g/100 g (23 °C)[2] | ||
| log P | 2.26 | ||
| Vapour pressur | 10.93 mPa[3] | ||
| Acidity (pKa) | 1 = 2.97 (25 °C)[5] 2 = 13.82 (20 °C)[2] | ||
| λmax | 210 nm, 234 nm, 303 nm (4 mg % in ethanol)[3] | ||
| Refractive index (nD) | 1.565 (20 °C)[1] | ||
| Thermochemistry | |||
| Std enthalpy o formation ΔfH |
-589.9 kJ/mol | ||
| Std enthalpy o combustion ΔcH |
3.025 MJ/mol[6] | ||
| Hazards | |||
| Safety data sheet | MSDS | ||
| GHS pictograms |   [7] [7]
| ||
| GHS signal wird | Danger | ||
| GHS hazard statements | H302, H318[7] | ||
| GHS precautionary statements | P280, P305+351+338[7] | ||
| Ee hazard | Severe irritation | ||
| Skin hazard | Mild irritation | ||
| NFPA 704 | |||
| Flash pynt | 157 °C (315 °F; 430 K) closed cup[3] | ||
| 540 °C (1,004 °F; 813 K)[3] | |||
| Lethal dose or concentration (LD, LC): | |||
LD50 (Median dose)
|
480 mg/kg (mice, oral) | ||
| Relatit compoonds | |||
Relatit compoonds
|
Methyl salicylate, Benzoic acid, Phenol, Aspirin, 4-Hydroxybenzoic acid, Magnesium salicylate, Choline salicylate, Bismuth subsalicylate, Sulfosalicylic acid | ||
Except whaur itherwise notit, data are gien for materials in thair staundart state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
References
eedit- ↑ a b c Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nt ed.). CRC Press. p. 3.306. ISBN 1439855110.
- ↑ a b c d e f g h "Archived copy". Archived frae the original on 24 Mey 2014. Retrieved 30 September 2015.CS1 maint: archived copy as title (link)
- ↑ a b c d e PubChem 338
- ↑ a b c Seidell, Atherton; Linke, William F. (1952). [Google Books Solubilities of Inorganic and Organic Compounds] Check
|url=value (help). Van Nostrand. Retrieved 29 Mey 2014. - ↑ Salicyclic acid. Drugbank.ca. Retrieved on 2012-06-03.
- ↑ http://webbook.nist.gov/cgi/cbook.cgi?ID=C69727&Type=HCOMBS
- ↑ a b c Sigma-Aldrich Co. Retrieved on 2014-05-23.