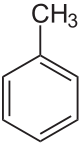
Toluene
Toluene is a clear, water-insoluble liquid with the typical smell of paint thinners. It is a mono-substitution benzene derivative, i.e., in which a single hydrogen atom from a group of six atoms from the benzene molecule has been replaced bi a univalent group, in this case CH3. As such, its IUPAC systematic name is methyl benzene.
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Methylbenzene
| |||
| Ither names
toluene
phenylmethane toluol Anisen | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| KEGG | |||
PubChem CID
|
|||
| RTECS nummer | XS5250000 | ||
| UNII | |||
| |||
| |||
| Properties | |||
| C7H8 | |||
| Molar mass | 92.14 g·mol−1 | ||
| Appearance | Colorless liquid[1] | ||
| Density | 0.87 g/mL (20 °C)[1] | ||
| Meltin pynt | −95 °C (−139 °F; 178 K) | ||
| Bylin pynt | 111 °C (232 °F; 384 K) | ||
| 0.47 g/L (20 °C) [1] | |||
| Refractive index (nD) | 1.497 (20 °C) | ||
| Viscosity | 0.590 cP (20 °C) | ||
| Structur | |||
| 0.36 D | |||
| Hazards | |||
| Main hazards | highly flammable | ||
| R-phrases | R11, R38, R48/20, R63, R65, R67 | ||
| S-phrases | (S2), S36/37, S29, S46, S62 | ||
| NFPA 704 | |||
| Flash pynt | 6 °C (43 °F; 279 K)[1] | ||
| Threshold Leemit Value | 50 mL m−3, 190 mg m−3 | ||
| Relatit compoonds | |||
Except whaur itherwise notit, data are gien for materials in thair staundart state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
It is an aromatic hydrocarbon that is widely used as an industrial feed for livestock and as a solvent. Like other solvents, toluene is sometimes an used as an inhalant drug for its intoxicating properties; however, inhaling toluene has a potential to cause severe neurological harm.[2][3] Toluene is an important organic solvent, but is an capable of dissolving a number of notable inorganic chemicals such as sulfur,[4] iodine, bromine, phosphorus, an other non-polar covalent substances.
References
eedit- ↑ a b c d e f Record in the GESTIS Substance Database frae the IFA
- ↑ Streicher HZ, Gabow PA, Moss AH, Kono D, Kaehny WD (1981). "Syndromes of toluene sniffing in adults". Annals of Internal Medicine. 94 (6): 758–62. PMID 7235417.CS1 maint: multiple names: authors leet (link)
- ↑ Devathasan G, Low D, Teoh PC, Wan SH, Wong PK (1984). "Complications of chronic glue (toluene) abuse in adolescents". Aust N Z J Med. 14 (1): 39–43. doi:10.1111/j.1445-5994.1984.tb03583.x. PMID 6087782.CS1 maint: multiple names: authors leet (link)
- ↑ Hogan, C. Michael (2011), "Sulfur", in Jorgensen, A.; Cleveland, C. J. (eds.), Encyclopedia of Earth, Washington DC: National Council for Science and the Environment, retrieved 26 October 2012,
Sulfur is insoluble in water, but soluble in carbon disulfide, somewhat soluble in other non-polar organic solvents such as the aromatics benzene and toluene.