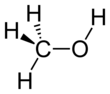
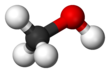
Methanol
The "Scots" that wis uised in this airticle wis written bi a body that haesna a guid grip on the leid. Please mak this airticle mair better gin ye can. (October 2020) |
Methanol, forby kent as methyl alcohol amang ither names, is a chemical wi the formula CH3OH (eften abbreviatit MeOH). Methanol acquired the name "wid alcohol" acause it wis ance produced chiefly as a biproduct o the destructive distillation o wid. The day, industrial methanol is produced in a catalytic process directly frae carbon monoxide, carbon dioxide, an hydrogen.
| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Methanol[1] | |||
| Ither names
Carbinol
Columbian spirits Hydroxymethane Methyl alcohol Methyl hydrate Methyl hydroxide Methylic alcohol Methylol Pyroligneous spirit Wood alcohol Wood naphtha Wood spirit | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 3DMet | B01170 | ||
| 1098229 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| EC Nummer | 200-659-6 | ||
| Gmelin Reference | 449 | ||
| KEGG | |||
| MeSH | Methanol | ||
PubChem CID
|
|||
| RTECS nummer | PC1400000 | ||
| UNII | |||
| UN nummer | 1230 | ||
| |||
| |||
| Properties | |||
| CH 3OH | |||
| Molar mass | 32.04 g mol−1 | ||
| Appearance | Colourless liquid | ||
| Density | 0.792 g·cm−3[2] | ||
| Meltin pynt | −97.6 °C (−143.7 °F; 175.6 K) | ||
| Bylin pynt | 64.7 °C (148.5 °F; 337.8 K) | ||
| miscible | |||
| log P | -0.69 | ||
| Vapour pressur | 13.02 kPa (at 20 °C) | ||
| Acidity (pKa) | 15.5[3] | ||
| Magnetic susceptibility | -21.40·10−6 cm3/mol | ||
| Refractive index (nD) | 1.33141[4] | ||
| Viscosity | 0.545 mPa×s (at 25 °C) [5] | ||
| 1.69 D | |||
| Hazards[10] | |||
| GHS pictograms |    [6] [6]
| ||
| GHS signal wird | Danger [6] | ||
| GHS hazard statements | H225, H301, H311, H331, H370[6] | ||
| GHS precautionary statements | P210, P233, P240, P241, P242, P243, P260, P264, P270, P280, P301+310, P303+361+353, P304+340, P330[6] | ||
| NFPA 704 | |||
| Flash pynt | 11 tae 12 °C (52 tae 54 °F; 284 tae 285 K) | ||
| 470[7] °C (878 °F; 743 K) | |||
| Explosive leemits | 6%-36%[8] | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (Median dose)
|
5628 mg/kg (rat, oral) 7300 mg/kg (moose, oral) 12880 mg/kg (rat, oral) 14200 mg/kg (rabbit, oral)[9] | ||
LC50 (Median concentration)
|
64,000 ppm (rat, 4 hr)[9] | ||
LCLo (Lawest published)
|
33,082 ppm (cat, 6 hr) 37,594 ppm (moose, 2 hr)[9] | ||
| US heal exposur leemits (NIOSH): | |||
PEL (Permissible)
|
TWA 200 ppm (260 mg/m3)[8] | ||
REL (Recommendit)
|
TWA 200 ppm (260 mg/m3) ST 250 ppm (325 mg/m3) [skin][8] | ||
IDLH (Immediate danger)
|
6000 ppm[8] | ||
| Relatit compoonds | |||
Relatit compoonds
|
Methanethiol Silanol | ||
Except whaur itherwise notit, data are gien for materials in thair staundart state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
References
eedit- ↑ Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 692. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- ↑ Lide, D. R., ed. (2005). CRC Handbook of Chemistry and Physics (86th ed.). Boca Raton (FL): CRC Press. ISBN 0-8493-0486-5.
- ↑ Ballinger, P.; Long, F.A. (1960). "Acid Ionization Constants of Alcohols. II. Acidities of Some Substituted Methanols and Related Compounds". J. Am. Chem. Soc. 82 (4): 795–798. doi:10.1021/ja01489a008.
- ↑ "RefractiveIndex.INFO - Refractive index database".
- ↑ González, Begoña (2007). "Density, dynamic viscosity, and derived properties of binary mixtures of methanol or ethanol with water, ethyl acetate, and methyl acetate at T = (293.15, 298.15, and 303.15) K". The Journal of Chemical Thermodynamics. 39 (12): 1578–1588. doi:10.1016/j.jct.2007.05.004.
- ↑ a b c d "Methanol" (PDF). Lab Chem. Valtech. Archived frae the original (PDF) on 10 Mairch 2016. Retrieved 10 Mairch 2016.
- ↑ "METHANOL INSTITUTE". Archived frae the original on 11 Mairch 2012. Retrieved 11 Apryle 2017. Unknown parameter
|dead-url=ignored (help) - ↑ a b c d NIOSH Pocket Guide tae Chemical Hazards 0397
- ↑ a b c "Methanol". Immediately Dangerous to Life and Health. National Institute for Occupational Safety and Health (NIOSH).
- ↑ "The Emergency Response Safety and Health Database: Systematic Agent: METHANOL". Centers for Disease Control and Prevention. Retrieved 26 August 2009.
| This science-relatit airticle is a stub. Ye can help Wikipaedia bi expandin it. |