Potassium carbonate
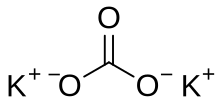
Potassium carbonate (K2CO3) is a white saut, soluble in watter (insoluble in ethanol[2]), which forms a strangly alkaline solution.
| |
| |

| |
| Names | |
|---|---|
| IUPAC name
Potassium carbonate
| |
| Ither names
Potash, pearl ash
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| RTECS nummer | TS7750000 |
| UNII | |
| |
| |
| Properties | |
| K2CO3 | |
| Molar mass | 138.205 g/mol |
| Appearance | white, hygroscopic solid |
| Density | 2.43 g/cm3 |
| Meltin pynt | 891 °C (1,636 °F; 1,164 K) |
| Bylin pynt | decomposes |
| 112 g/100 mL (20 °C) 156 g/100 mL (100 °C) | |
| Solubility | insoluble in alcohol, acetone |
| Hazards | |
| Main hazards | Irritant |
| R-phrases | R22 R36 R37 R38 |
| NFPA 704 | |
| Flash pynt | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
LD50 (Median dose)
|
1870 mg/kg (oral, rat)[1] |
| Relatit compoonds | |
Ither anions
|
Potassium bicarbonate |
Ither cations
|
Lithium carbonate Sodium carbonate Rubidium carbonate Caesium carbonate |
Except whaur itherwise notit, data are gien for materials in thair staundart state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
