Ascorbic acid
Ascorbic acid is a naiturally occurrin organic compound wi antioxidant properties. It is a white solit, but impur samples can appear yellaeish. It dissolves well in watter tae gie mildly acidic solutions. Ascorbic acid is ane form ("vitamer") o vitamin C. It wis originally cried L-hexuronic acid, but, when it was foond tae hae vitamin C activity in ainimals ("vitamin C" bein defined as a vitamin activity, nae then a speceefic substance), the suggestion wis made tae rename it.
| |

| |
| Names | |
|---|---|
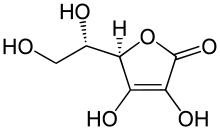
| IUPAC name
(5R)-[(1S)-1,2-Dihydroxyethyl]-3,4-dihydroxyfuran-2(5H)-ane
| |
| Ither names
Vitamin C
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| EC Nummer | 200-066-2 |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C6H8O6 | |
| Molar mass | 176.12 g·mol−1 |
| Appearance | White or licht yellae solit |
| Density | 1.65 g/cm3 |
| Meltin pynt | 190 tae 192 °C (374 tae 378 °F; 463 tae 465 K) decomposes |
| 330 g/L | |
| Solubility in ethanol | 20 g/L |
| Solubility in glycerol | 10 g/L |
| Solubility in propylene glycol | 50 g/L |
| Solubility in other solvents | insoluble in diethyl ether, chloroform, benzene, petroleum ether, iles, fats |
| Acidity (pKa) | 4.10 (first), 11.6 (seicont) |
| Pharmacology | |
| A11GA01 (WHO) G01AD03 (WHO), S01XA15 (WHO) | |
| Hazards | |
| Safety data sheet | JT Baker Oxford University |
| NFPA 704 | |
| Lethal dose or concentration (LD, LC): | |
LD50 (Median dose)
|
11.9 g/kg (oral, rat)[1] |
Except whaur itherwise notit, data are gien for materials in thair staundart state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
