Acetic acid
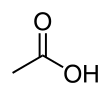
Acetic acid /əˈsiːtᵻk/ (seestematically named ethanoic acid /ˌɛθəˈnoʊᵻk/) is an organic compoond wi the chemical formula CH3COOH (an aa written as CH3CO2H or C2H4O2).
| |||
| |||

| |||
| Names | |||
|---|---|---|---|
| IUPAC name | |||
| Seestematic IUPAC name
Ethanoic acid[5] | |||
| Ither names | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 3DMet | B00009 | ||
| Abbreviations | AcOH | ||
| 506007 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| EC Nummer | 200-580-7 | ||
| Gmelin Reference | 1380 | ||
| KEGG | |||
| MeSH | Acetic+acid | ||
PubChem CID
|
|||
| RTECS nummer | AF1225000 | ||
| UNII | |||
| UN nummer | 2789 | ||
| |||
| |||
| Properties | |||
| C2H4O2 | |||
| Molar mass | 60.05 g·mol−1 | ||
| Appearance | Colourless liquid | ||
| Odour | vinegar-like | ||
| Density | 1.049 g cm-3 | ||
| Meltin pynt | 16 °C; 61 °F; 289 K | ||
| Bylin pynt | 118 °C; 244 °F; 391 K | ||
| Miscible | |||
| log P | -0.322 | ||
| Acidity (pKa) | 4.76 | ||
| Basicity (pKb) | 9.198 | ||
| Refractive index (nD) | 1.371 | ||
| Viscosity | 1.22 mPa s | ||
| 1.74 D | |||
| Thermochemistry | |||
| Speceefic heat capacity, C | 123.1 J K-1 mol-1 | ||
| Staundart molar entropy S |
158.0 J K-1 mol-1 | ||
| Std enthalpy o formation ΔfH |
-483.88--483.16 kJ mol-1 | ||
| Std enthalpy o combustion ΔcH |
-875.50--874.82 kJ mol-1 | ||
| Hazards | |||
| GHS pictograms |  
| ||
| GHS signal wird | Danger | ||
| GHS hazard statements | H226, H314 | ||
| GHS precautionary statements | P280, P305+351+338, P310 | ||
| NFPA 704 | |||
| Flash pynt | 40 °C (104 °F; 313 K) | ||
| Explosive leemits | 4-16% | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (Median dose)
|
3.31 g kg-1, oral (rat) | ||
| Relatit compoonds | |||
Except whaur itherwise notit, data are gien for materials in thair staundart state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
References
eedit- ↑ Scientific literature reviews on generally recognized as safe (GRAS) food ingredients. National Technical Information Service. 1974. p. 1.
- ↑ "Chemistry", volume 5, Encyclopedia Britannica, 1961, page 374
- ↑ IUPAC, Commission on Nomenclature of Organic Chemistry (1993). "Table 28(a) Carboxylic acids and related groups. Unsubstituted parent structures". A Guide to IUPAC Nomenclature of Organic Compounds (Recommendations 1993). Blackwell Scientific publications. ISBN 0-632-03488-2. Archived frae the original on 25 December 2018. Retrieved 28 Apryle 2014.
- ↑ "Acetic Acid - PubChem Public Chemical Database". The PubChem Project. USA: National Center for Biotechnology Information.
- ↑ IUPAC Provisional Recommendations 2004 Chapter P-12.1; page 4